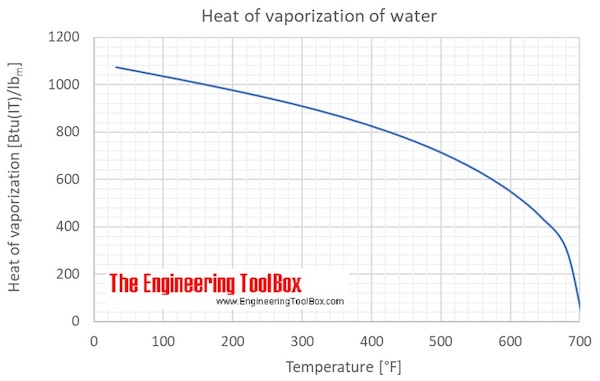
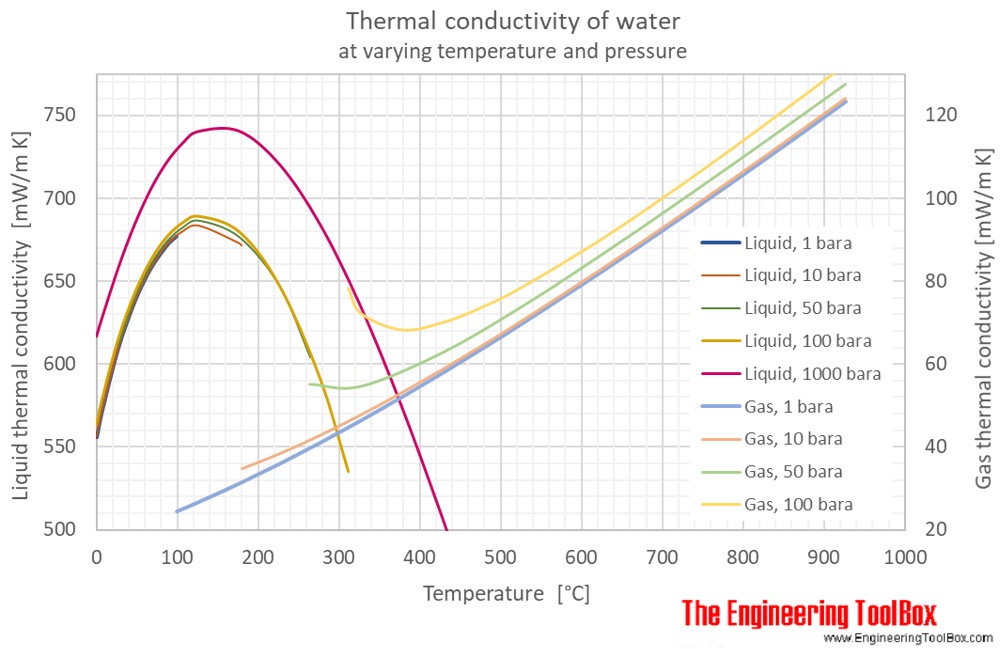
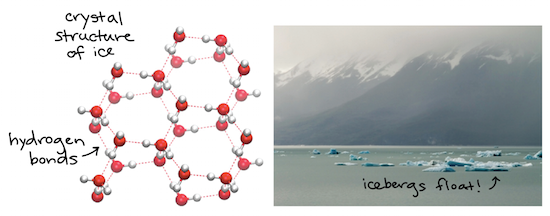
Water is one of the latter it has a high specific heat capacity because it requires more energy to raise the temperature.
Specific heat of water at room temperature approximately is.
Water specific heat online calculator figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to 360 c 32 700 f si and imperial units.
As with most liquids the temperature of water increases as it absorbs heat and decreases as it releases heat.
This means that it takes 4 200 j to raise the temperature of 1 kg of water by 1 c.
A brief summary of the procedure is outlined below.
The specific heat capacity c p of liquid water at room temperature and pressure is approximately 4 2 j g c.
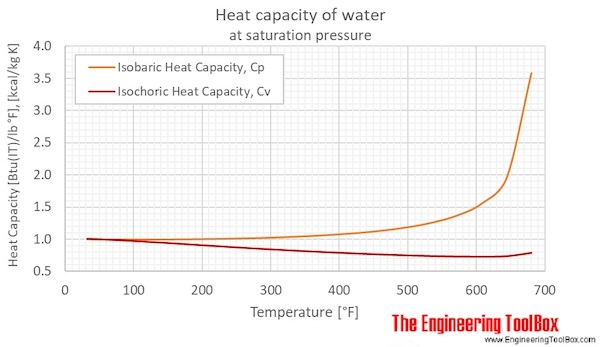
We will consider the specific heat capacity of the water to be known 1 00 kcal kg k.
The specific heat capacity symbol c p of a substance is the heat capacity of a sample of the substance divided by the mass of the sample.
The procedure for this experiment is thoroughly covered in the coinciding specific heat test article.
The following table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials and when applicable the molar heat capacity.
This solution uses 0 901 for aluminum and 4 18 for water.
For water vapour at room temperature and pressure the value of specific heat capacity cp is approximately 1 9 j g c.
Since specific heat capacity does not depend on the object in question only the substance from which it is made specific heat capacities are much more useful.
This means it takes 4 2 joules of energy to raise 1 gram or 1 milliliter if you d rather think of the equivalent volume of 1 gram of water of water by 1 degree celsius.
The specific heat capacity of water vapour at room temperature is also higher than most other materials.
A mass of water was measured than poured into the calorimeter the water remained there until it reached room temperature.
Because water is such an important and common substance we even have a special way to identify the amount of energy it takes to raise one gram of water by one degree.
Generally the most constant parameter is notably the volumetric heat capacity at least for solids which is notably around the value of 3 megajoule per cubic meter and kelvin.
You need to look up the specific heat values c for aluminum and water.
Water has a specific heat capacity of 4182 j kg c.
Again you use q mcδt except you assume q aluminum q water and solve for t which is the final temperature.
Units of heat btu calorie and joule the most common units of heat are btu british thermal unit calorie and joule.